Our Goals:
Neurological and Psychiatric disorders pose a major socioeconomic burden. Our goal is to determine how genetic mutations underlie the pathobiology of nervous system disorders. We take advantage of cutting edge genomic technologies to study the human brain at unprecendented resolution. We specifically seek to :
1. Identify molecular programs regulating cell type distinctions in the human nervous system, and to identify changes in disease;
2. Uncover the regulatory networks that control stem cell differentiation that could be utilized to increase the precision and safety of stem cell - based therapies;
3. Develop new experimentally tractable model of human disease that enable interrogation of disease mechanisms and discovery of new therapeutic opportunities.
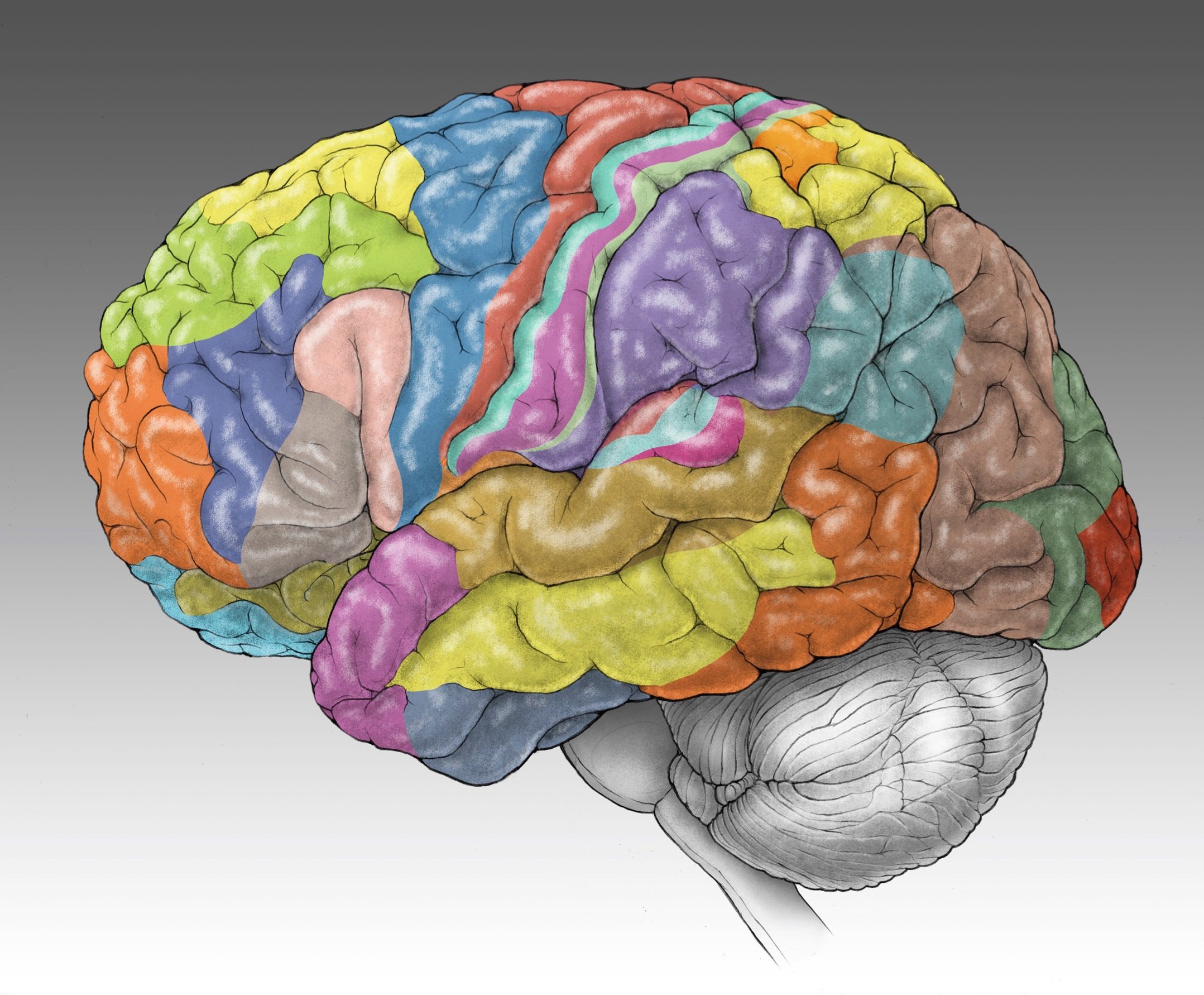
Cerebral cortex is subdivided into dozens of anatomical and functional areas. Our goal is to uncover how these regions emerge during development. To learn more, see Cadwell et a. 2019 Neuron PMID: 31557462
Thalamus is a centrally located brain structure involved in a wide array of sensory, motor, and cognitive functions. To learn more, see Kim et al. 2023 Science PMID: 37824646
Representative Projects and Contributions to Science
Developing tools for human neuroscience
We have a long-standing interest in deciphering how cells of the human brain function in health and disease. Harnessing technologies that allow us to manipulate cells in the human brain have the potential to become next-generation treatments for brain disorders.
Key publications:
Andrews, J. P., et al. (2024). Multimodal evaluation of network activity and optogenetic interventions in human hippocampal slices. Nature Neuroscience. doi.org/10.1038/s41593-024-01782-5.
Lee AT, Chang EF, Paredes MF, Nowakowski TJ. Large-scale neurophysiology and single-cell profiling in human neuroscience. Nature. 2024 Jun;630(8017):587-595. doi: 10.1038/s41586-024-07405-0. Epub 2024 Jun 19. PMID: 38898291
Zhu D, Brookes DH, Busia A, Carneiro A, Fannjiang C, Popova G, Shin D, Donohue KC, Lin LF, Miller ZM, Williams ER, Chang EF, Nowakowski TJ, Listgarten J, Schaffer DV. Optimal trade-off control in machine learning-based library design, with application to adeno-associated virus (AAV) for gene therapy. Sci Adv. 2024 Jan 26;10(4):eadj3786. doi: 10.1126/sciadv.adj3786. Epub 2024 Jan 24. PMID: 38266077
Adapted, from Andrews, J. P., et al. (2024) PMID: 39548326
Epigenetic mechanisms of brain development
Epigenetics is the study of how gene expression is regulated. Because genes are essential to the development of the brain, subtle differences in cis- and trans- factors can have significant impact on how our brains develop. This is important because non-coding mutations in the DNA can increase or decrease susceptibility to certain neurodevelopmental disorders, and because mutations in genes that encode proteins which regulate the expression of other genes can lead to severe neurodevelopmental or psychiatric symptoms. We seek to uncover the molecular mechanisms of gene expression regulation through functional genomics to better understand how epigenetic factors contribute to brain development and susceptibility to neurodevelopmental disorders.
Key publications:
Ziffra et al. (2021) Single-cell epigenomics reveals mechanisms of human cortical development. Nature. PMID: 34616060
Deng C, et al. (2024) Massively parallel characterization of regulatory elements in the developing human cortex. Science. PMID: 38781390
Wang et al. preprint A foundational atlas of autism protein interactions reveals molecular convergence. bioRxiv https://doi.org/10.1101/2023.12.03.569805
Single cell ATAC sequencing reveals epigenetic mechanisms of brain development, including early signatures of cortical arealization among progenitor cells. Adapted, from Ziffra et al. 2021. PMID: 34616060
Development of the thalamus
Thalamus was once called a ‘gateway’ to the cerebral cortex, because it transmits information from the body to the higher order brain structures, including the cerebral cortex. However, its development remains poorly understood, as studies of neural development have focused overwhelmingly on the cerebral cortex. By applying unbiased genomic technologies, spatial transcriptomics, and in vitro modeling, we seek to uncover the rules by which progenitors of the thalamus give rise to thalamic nuclei, as well as axonal projections to the cerebral cortex. We are also using in vivo and ex vivo experimental models to reveal the effects of rare neuropsychiatric risk variants on the development of the thalamus and its interactions with other brain structures.
Key publications:
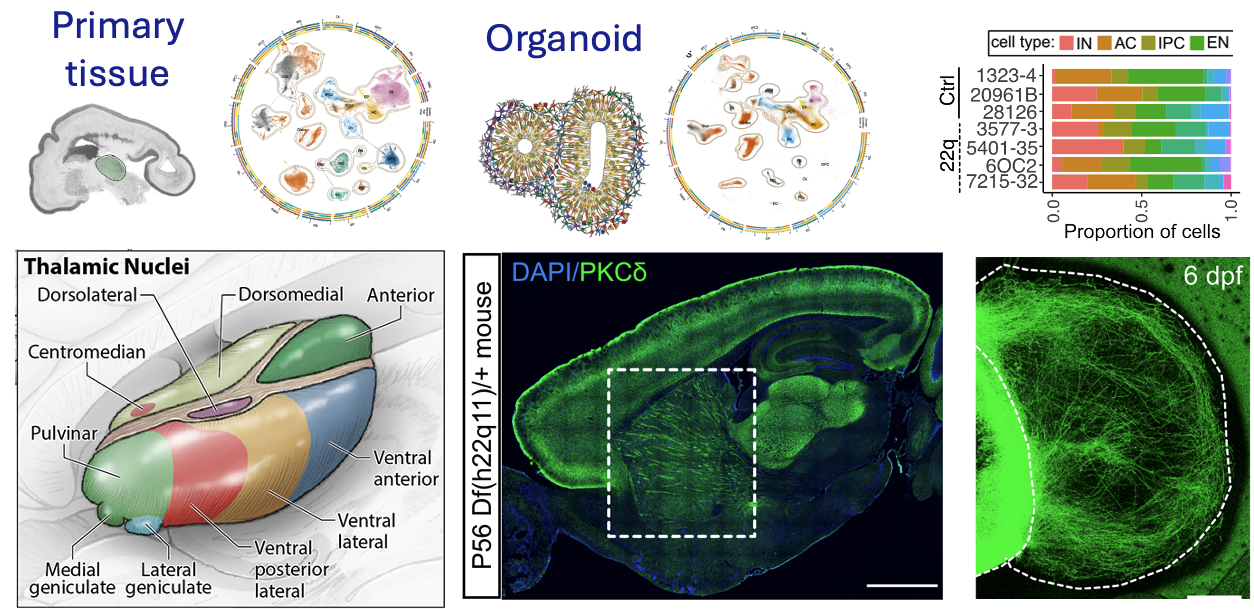
Shin D, et al. (2024) Thalamocortical organoids enable in vitro modeling of 22q11.2 microdeletion associated with neuropsychiatric disorders. Cell Stem Cell PMID: 38382530
Kim CN et al. (2023) Spatiotemporal molecular dynamics of the developing human thalamus. Science doi: 10.1126/science.adf9941
Thalamus is a centrally located brain structure involved in many fundamental sensory, motor, and cognitive processes, yet its development and vulnerability to genetic mutations are rarely studied. We apply genomic technologies to uncover the fundamental rules by which cells in this brain region emerge during development and establish interactions with the cerebral cortex. We are interested in studying how genetic mutations lead to abnormal development of this pathway using in vivo and ex vivo models. Adapted, from Shin et al. 2024 PMID: 38382530
Developmental lineage relationships in the brain
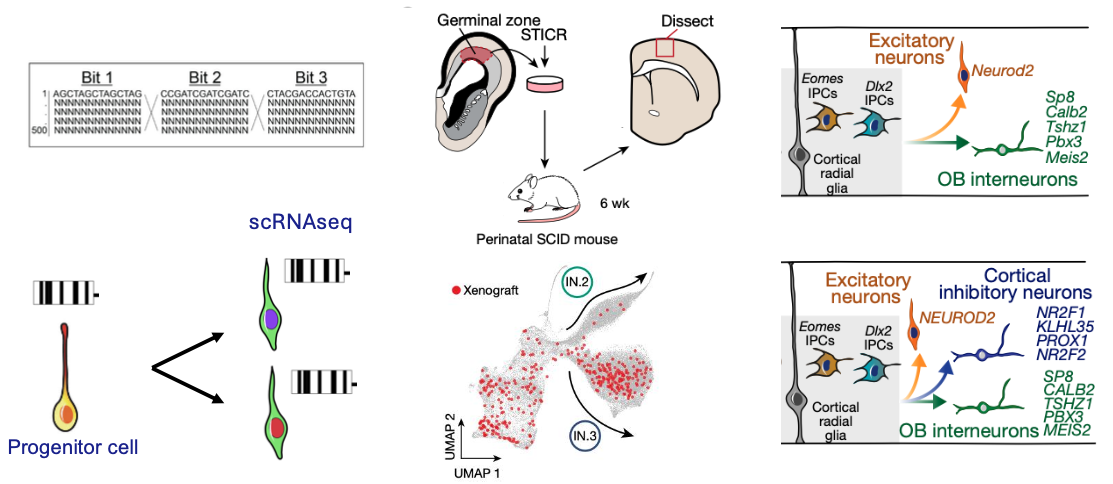
The adult human brain contains billions of neurons and glia that can be categorized into thousands of distinct types. How this amazing complexity emerges from a limited set of progenitor cells remains a fundamental question in developmental neurobiology. We apply massively parallel molecular barcoding strategies to uncover what cell types are generated at different stages of development, from which progenitor types, and in what order. Developmental lineage represents a hidden map of interdependencies that underlies the map of cellular diversity, and we seek to uncover the fundamental rules by which the nervous system forms during development.
Key publications:
Allen DE, et al. 2022 Fate mapping of neural stem cell niches reveals distinct origins of human cortical astrocytes. Science DOI: 10.1126/science.abm5224
Delgado RN, et al. 2022 Individual human cortical progenitors can produce excitatory and inhibitory neurons. Nature. PMID: 34912114
Developmental cell lineage can be traced using molecular barcoding of progenitor cells. We have shown that cortical progenitor cells can give rise to GABAergic neurons in addition to Glutamatergic neurons. Adapted, from Delgado et al. 2022 PMID: 34912114
Neuroimmune interactions in developing brain and disease
Although immune cells are not abundant in the brain, they play important roles in protecting the developing brain from viral infections. In addition, presence and abnormal activation of immune cells can compromise the delicate homeostasis of tissues and underlie neurological complications.
Key publications:
Popova G, et al. (2023). Rubella virus tropism and single cell responses in human primary tissue and microglia-containing organoids. eLife PMID: 37470786
Winkler EA, et al. (2022) Single cell atlas of the normal and malformed human brain vasculature. Science doi: 10.1126/science.abi7377.
Popova G, et al. (2021) Human microglia states are conserved across experimental models and regulate neural stem cell responses in chimeric organoids Cell Stem Cell. PMID: 34536354
Microglia in developing brain a primary cellular target of viral infections. These interactions can be modeled using chimeric brain organoids. Immune cells play essential roles in neurological disorders, and their abnormal activation can increase the risk of arteriovenous malformation rupture. Adapted from Popova et al. 2023 PMID: 37470786 and Winkler et al. 2022 PMID: 35084939
Collaborative Research
We are excited to actively participate in the following consortia efforts:
In the news:
Pulses of light show promise in controlling seizures
New Map of Brain Vasculature Reveals Unexpected Diversity
We Can Now See the Brain Like Never Before
Building a brain: How does it generate its exquisite diversity of cells?
Vilcek Prize for Creative Promise in Biomedical Science
Newly Discovered Immune Cells Play a Role in Hemorrhagic Stroke
Funding sources
We are grateful for the generous support of our sponsors: